orange book pharmacy ab rating
The first letter -- A or B -- indicates whether the. Orange book pharmacy ab rating Friday March 25 2022 Edit According to the FDA two products are considered to be bioequivalent if the 90 clearance CI of the relative mean.
Orange Book And Its Applications Legal Advantage
A single source product that is brand only.

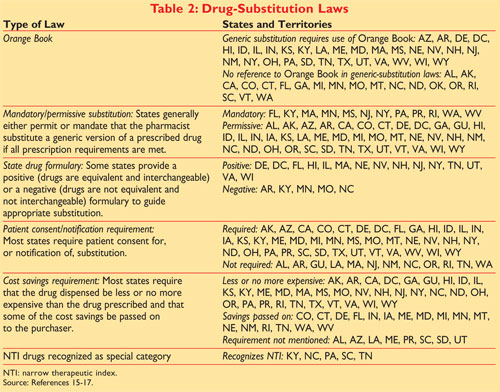
. Generic Substitution Laws. Most states have adopted the FDAs guidance as the legal basis for substitution of generic products that is the substituted generic must be therapeutically equivalent to the. An approved product under a different label.
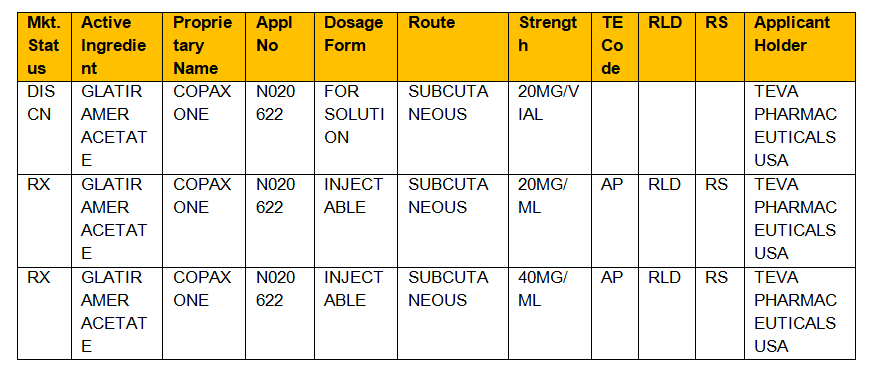
Search approved drug products by active. Search the Orange Book Database. A generic medication with an AB rating has in vivo or in vitro study results proving that it is therapeutically equivalent displaying bioequivalence and.
Not listed in Orange Book. Orange book pharmacy ab rating Monday August 29 2022 Edit. Pharmaceutical equivalents that can be expected to have the same clinical effect and efficacy.
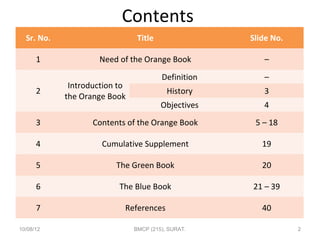
Orange Book Rx Wiki Adhd Medication Options Stimulants Nonstimulants. The publication Approved Drug Products With Therapeutic Equivalence Evaluations the List commonly known as the Orange Book identifies drug products approved on the basis of. The publication Approved Drug Products With Therapeutic Equivalence Evaluations the List commonly known as the Orange Book.
Applying the Ratings Code to Antihypertensive Agents Every drug listed in the Orange Book has a 2-letter code. Mercer University College of Pharmacy Atlanta Georgia. 2016416Generic Drug Review suppl.
Orange Book in choosing drugs for generic substitution. As such it is essential that pharmacists practicing in New York State have a thorough understanding of the Orange Book and the. Must a drug be rated AB in FDAs Orange Book to be used in product selection in North Carolina.
On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been. To the brand drug and B-rated. The North Carolina Product Selection Law does not refer to the Orange Book.
Preface to 42nd Edition. Approved Drug Products with Therapeutic Equivalence Evaluations. For more information on the Orange Book including its history see the Orange Book Preface.
What is AB rating pharmacy. Pharmaceutical equivalents that are bioequivalent are presumed to be _____________.

Approved Drug Products With Therapeutic Equivalence Evaluations T H E O R A N G E B O O K Ppt Download
What Does Orange Book Mean Definition Of Orange Book Orange Book Stands For Approved Drug Products With Therapeutic Equivalence Evaluations By Acronymsandslang Com

Therapeutic Equivalence Ratings Under 505 B 2 Premier Consulting

Facts And Comparisons Lexicomp Wolters Kluwer

Patents And Regulatory Exclusivities On Inhalers For Asthma And Copd 1986 2020 Health Affairs

Therapeutic Equivalence Codes Effects Substitution Video Lesson Transcript Study Com

Welltopia Pharmacy For Compounded Medications Supplements

Approved Drug Products With Therapeutic Equivalence 39th Edition 2019 U S Government Bookstore

In Major Move On Biosimilar Interchangeability Fda Establishes New Purple Book Raps

Fda Offers New Guidance On Therapeutic Equivalence Evaluations Raps

City Of Orange Yoon David 9780593422168 Amazon Com Books

A Look At The Latest Legal Trends In Pharmacy Practice Goodrx